HBW Insight
Volleyball great Gabby Reece promotes Stemregen Sport; eye care pharma Harrow changes scientific lead; sisters partner to launch Renew-V vaginal moisturizer; Haleon tabs former Unilever executive as chief customer officer; and former Bayer executive leads Perrigo’s expanded scientific office.
AHPA’s significant legislative victories included enactment of Dietary Supplement and Nonprescription Drug Consumer Protection Act in 2006 to establish a serious adverse event reporting law for vitamin, mineral and supplement products.
Ulta Beauty, Inc. CEO Dave Kimbell retires after 11 years with the beauty retailer and is replaced by president and chief operating officer Kecia Steelman, as a strong holiday sales period prompts the firm to update its fiscal fourth quarter projections.
In response to an IPA Europe complaint, European Ombudsman Emily O’Reilly finds that the Commission’s interpretation of EU food legislation in relation to probiotics is “reasonable and in line with the main goal of this legislation, which is to ensure a high level of consumer protection.”
Final rule’s requirement that ACNU formulations also remain available as Rx generics could limit revenues for marketers of the nonprescription drugs while approvals of ACNU applications from studies with participants having drugs delivered to their homes may require marketers to limit distribution to direct-to-consumer without clearance for sales in retail stores.
Anti-aging skin care device marketer UVVU Inc. agreed to discontinue or modify a number of claims for its SolaWave 4-in-1 Skin Care Wand with Red Light Therapy, since statements were not substantiated by an in-home use study on which they relied, says the National Advertising Division.
A round-up of the latest European consumer health appointments: Maxwellia adds CFO and board member; Reckitt restructures operations; Barentz names CEO.
Study completed in 2024 showed consumers using an online application accurately self-selected and safely used an Rx statin drug. An “ACNU” switch NDA would have to first show conventional OTC labeling won’t support proper self-selection and use.
L’Oréal Group unveils Cell BioPrint, a hardware device that provides personalized skin analysis in just five minutes, while Shiseido and Samsung win innovation awards for their digital beauty devices during the Consumer Technology Association’s Consumer Electronics Show in Las Vegas.
Perrigo's retail customers are expecting real "net zero" progress from consumer health manufacturers, which is in turn driving the firm's sustainability ambitions and expectations from their own suppliers, Perrigo’s UK ethical compliance lead Isobel Gay tells HBW Insight in this exclusive interview.
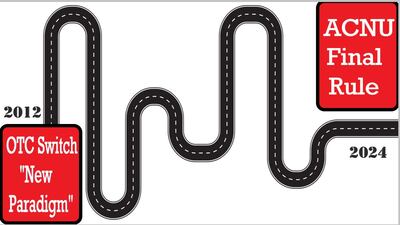
Agency’s proposal for targeting making more formulations available nonprescription through “novel switches” went through changes on its path from idea in 2012 to proposed rule in 2022 and final rule published in December.
FDA’s ‘ACNU’ rule went through numerous twists and turns from agency’s “new paradigm” idea of OTC switches in 2012 to publication.
Haleon now holds 88% of its China OTC joint venture after acquiring a larger stake in the business from its local partners.
National Advertising Division found multiple faults with Oral Essentials’ evidence for claim, including that an in vitro study the firm conducted on “mouse cells is not dispositive of whether the same product would have any adverse effects on humans under real world conditions.”
FDA publishes the first proposed rule under the Modernization of Cosmetics Regulation Act – a testing method for detecting asbestos in talc-containing cosmetics.
PAGB CEO Michelle Riddalls and ISF president emeritus David Webber have been awarded OBEs for their services to consumer health and self-care.
Hiya has more than 200,000 customers in the direct-to-consumer channel, all using subscriptions ordered on the brand’s website, and plans to expand into online marketplaces and retail chains. The startup “offers a compelling subscription model with attractive margins, profitability, and strong cash flow generation,” says USANA CEO Jim Brown.
FDA Office of Nonprescription Drugs explains in its proposed commitment letter for fiscal years 2026-2030, based in part on suggestions by stakeholders, that it will heighten its attention to collecting those fees.
The Washington State Department of Ecology publishes ‘Interim Policy on Lead in Cosmetics’ which provides safe harbor options for cosmetic products struggling with the 1ppm limit under the state’s Toxic Free Cosmetics Act, while the department gathers information under a newly opened rulemaking to ‘identify a feasible approach to regulating lead in cosmetic products.’
Vitamin, mineral and supplement products “may bear nutrient content claims, including ‘healthy,’’ if they meet structure/function claim criteria “without being subject to the requirements of the ‘healthy nutrient content claim” stated in the final rule FDA published recently.